-
Xinxintian Industrial Zone, Shajing Street,Bao'an District, Shenzhen, China

Medical Device PCBA: High Standards & Regulatory Adherence
[Introduction]
In the field of electronic manufacturing services (EMS), the production of medical device PCBA is not only a technology-intensive job, but also a core link related to life safety. From operating rooms to home care scenarios, the reliability of medical equipment directly depends on the precision and compliance of PCBA. This article will deeply analyze the key manufacturing standards, technical challenges and future trends of medical device PCBA, and provide a systematic reference for industry practitioners.
1. The core definition and value positioning of medical device PCBA
Medical device PCBA is a functional component that integrates electronic components (such as sensors, microprocessors, capacitors, etc.) onto a multi-layer printed circuit board (PCB) through surface mount technology (SMT) and through-hole insertion technology. Compared with consumer electronics PCBA, medical-grade products need to meet three differentiated requirements:
- Zero defect tolerance standard: Fault tolerance approaches 0, and a single failure may cause serious consequences;
- Adaptability to extreme environments: Stable operation is required under the temperature range of -55℃ to 125℃, 85% humidity and high-frequency vibration conditions;
- Multi-dimensional compliance certification: International standards such as ISO 13485, FDA Class II/III, and IEC 60601 must be met simultaneously.
2. Six core manufacturing characteristics of medical device PCBA
| Characteristic dimensions | Technical requirements examples | Industry standard association |
|---|---|---|
| High reliability | 10^6 cycles without failure rate, MTBF≥100,000 hours | IEC 60601-1:2012 |
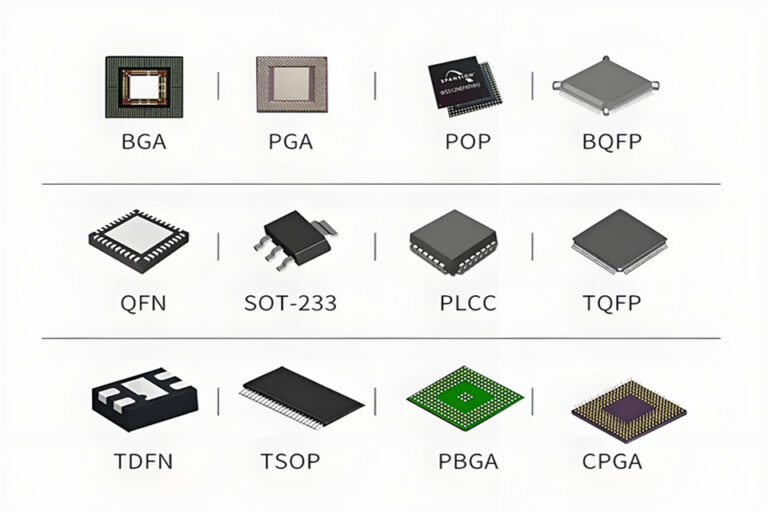
| Miniaturization design | 01005 chip mounting accuracy ±0.025mm, wiring spacing ≤3mil | IPC-A-610E Class 3 |
| Multi-layer complex structure | 12-20 layers of HDI board, micro blind hole diameter ≤6mil | IPC-2581 |
| Low power optimization | Standby current of wearable devices <1μA | AAMI ECRI 2020 |
| Anti-interference ability | EMI shielding effectiveness ≥60dB, signal integrity error <0.5% | ISO 80601-2-10 |
| Traceability | Each PCBA needs to keep 30 years of production data records | FDA 21 CFR Part 11 |
3. Regulatory compliance framework analysis
Medical device PCBA needs to pass a multi-dimensional compliance certification matrix:
- Quality management system: ISO 13485 requires the establishment of a full-process system covering design and development, procurement control, and production verification;
- Environmental directive: RoHS 3.0 restricts 10 types of hazardous substances, and REACH regulations manage 4500+ chemicals;
- Electrical safety standards: IEC 60601-1 stipulates that leakage current is less than 10μA and insulation resistance is greater than 100MΩ;
- Production specifications: GMP requires the cleanliness of the production environment to meet ISO Class 7 standards;
- Data compliance: FDA 21 CFR Part 11 stipulates that electronic records must be tamper-proof.
4. Three core challenges and solutions for medical device PCBA
1. Zero-defect manufacturing problem
- Problem: IEC 60601 requires a defect rate of <1ppm, and traditional detection methods are difficult to cover all defect types;
- Solution:
- Introduce a six-fold detection system (SPI solder paste detection, 3D AOI, X-Ray, boundary scan test, etc.);
- Use machine learning algorithms to optimize the detection model, and the defect recognition accuracy rate is increased to 99.99%.
2. Miniaturization and high-density integration
- Requirement example:
- Capsule endoscope PCBA needs to integrate 200+ components on an 8mm×8mm substrate;
- ECG monitoring bracelet needs to achieve 7 days of continuous monitoring with a 100mAh battery life.
- Technological breakthrough:
- Apply 0.8mm pitch BGA package to support 01005 chip (0.4mm×0.2mm) mounting;
- Develop high TG value (≥170℃) prepreg to improve the thermal stability of multilayer boards.
3. Extreme environment reliability verification
- Test plan:
- Temperature cycle test (-55℃↔125℃, 500 cycles);
- Salt spray test (5% NaCl solution, 96 hours of no corrosion);
- Mechanical shock test (half-sine wave, peak acceleration 500G).
5. Technical adaptation of core application scenarios
| Equipment type | PCBA key performance indicators | Typical technical solutions |
|---|---|---|
| CT/MRI signal board | 10Gbps high-speed transmission, crosstalk <-40dB | 10-layer HDI board, differential pair spacing ≥5mil |
| Ventilator control board | 1ms response delay, power ripple <5mV | 4-layer rigid-flexible board, DC-DC conversion efficiency >90% |
| Smart insulin pump | Bluetooth 5.0 communication, standby current <0.5μA | Ultra-low power MCU+dynamic voltage regulation technology |
6. Future technology trends and industry insights
- AI empowers manufacturing:
- Use computer vision for defect prediction to reduce manual re-inspection time by 30%;
- Process simulation based on digital twins to shorten the new product introduction cycle.
- Material innovation:
- Develop high temperature resistant (≥150℃) polyimide substrates to extend the life of the device;
- Research biocompatible packaging materials to reduce the risk of rejection of implantable devices.
- Edge computing integration:
- Integrate NPU (neural network processing unit) in PCBA to achieve localized AI diagnosis;
- 5G module integration makes the response delay of remote medical equipment less than 10ms.
7. Future prospects of medical device PCBA manufacturing
With the popularization of AI-assisted diagnosis and telemedicine, medical devices are evolving from “feature machines” to “smart terminals”. For example, smart insulin pumps need to be linked to cloud blood sugar data through wireless modules, which puts higher requirements on PCBA‘s communication performance and power consumption. In the future, medical device PCBA manufacturing will develop in the following directions:
- Higher integration: Through different packaging technologies, sensors, processors, and memories are integrated into a single module to reduce the size of the device.
- More stringent reliability: Develop new materials that are resistant to high temperatures and corrosion to adapt to more complex medical environments.
- Smarter functions: Integrate edge computing capabilities to achieve real-time data analysis and decision-making on the device side.

8. FAQ
Q1: What are the main differences between medical device PCBA and industrial-grade PCBA?
A: Medical device PCBA needs to meet stricter electrical safety standards (such as IEC 60601-1), higher environmental adaptability requirements (-55℃ to 125℃ operating range) and more complex compliance certification processes.
Q2: How to choose a medical PCBA manufacturer?
A: Prioritize whether the company holds ISO 13485 certification, FDA registration number, and has a medical-grade SMT production line (supporting 01005 component placement) and six-fold testing capabilities.
Q3: What is the minimum batch size for medical device PCBA?
A: Usually the minimum order is 500-1000 sets. Special customized products need to evaluate the mold development cost in advance.
Q4: What are the steps in the inspection process of medical PCBA?
A: Including SPI solder paste inspection, 3D AOI, X-Ray solder joint perspective, functional testing, salt spray test and mechanical vibration test.
Q5: How to achieve low power design in medical PCBA?
A: Use ultra-low power MCU, high-efficiency power management IC and dynamic voltage regulation technology to extend the battery life of wearable devices.
Q6: What is the future technology trend of medical PCBA?
A: Integrate AI chips, 5G communication modules and edge computing capabilities to promote the development of medical equipment towards intelligence and remoteness.
[Conclusion]
The manufacturing of medical device PCBA is not only the epitome of precision electronic engineering, but also the intersection of medical safety and technological innovation. With the penetration of technologies such as AI and 5G, medical device PCBA will develop towards higher integration and more intelligence. Companies need to continue to invest in R&D and build full-chain capabilities covering design, manufacturing, and verification in order to cope with increasingly stringent industry standards.